Space for a company committed to developing long-lasting, life-improving cancer treatments.
A muti-phase build-out of a research and manufacturing facility to support the development of clinical-stage cell therapy to treat cancer.
Key Partners
- BAM Creative
- FISKAA
- HATCHSpaces

$10,000,000
CONTRACT VALUE
18 months
SCHEDULE
49,200
SQUARE FEET
2022
YEAR COMPLETED
300
LINEAR FEET OF CO2 AND COMPRESSED AIR PIPING
Instil Bio is a fast-growing global pharmaceutical company in need of additional space and facilities. HITT was engaged to expand Instil Bio’s footprint in the greater Los Angeles area with a new multi-phase lab and support space build-out.
Client Goals
Following their IPO, Instil Bio tripled in size. The company’s rapid growth meant more facilities were needed to support its expanding operations and clinical trials. Previously only providing office space, Instil Bio expanded to include multiple programmatic elements, including space for clinical trials, warehouse, storage, research and development, office, and support spaces.
Design Vision
The design provides efficient use of the space while accommodating the various programming elements. The design intent balances an ever-changing programming background for a company that continued to grow aggressively through the duration of construction.
Construction Focus
This project shows a commitment from many different project team stakeholders to come together and deliver on a high-demand space. The project was completed with heavy overtime requirements to meet the client’s expedited timeline. The space is critical to the company’s clinical operations to support its continued growth and success.
Supporting New Clinical Requirements
The project’s second phase included the build-out of an ISO 8 cleanroom setup to support new clinical program requirements. The team implemented compatible materials that fit design requirements while procuring and delivering them in a timely manner to meet critical milestones.
Cold Room and GMP Storage
Our team built out an 8,000-sf warehouse to support a high-density racking system, cold room enclosure, and GMP storage. We consistently and carefully coordinated with the client’s on-site warehouse team to ensure rack layouts met their growing needs for the storage space.
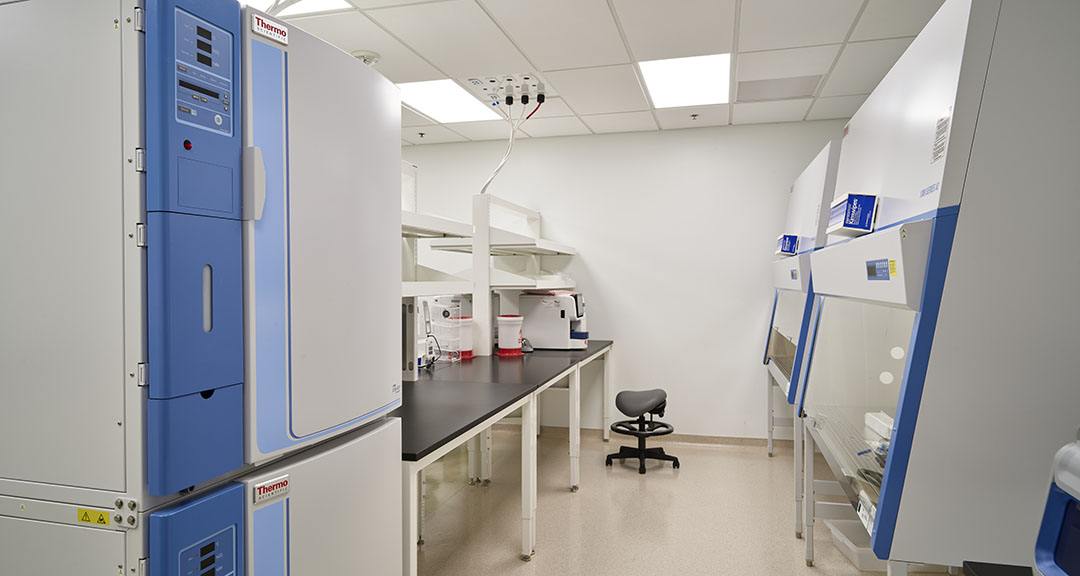
10,000-sf Wet Laboratory
HITT built out a fully commissioned wet lab space while the GMP facility remained fully active throughout construction. The team was hyper-focused on cleanliness, preparedness, and communication to provide extra attention to detail, and utilized lessons learned and knowledge of Instil Bio’s procedures to accelerate the timeline for this phase.
The key to success on this project was our team’s responsiveness, flexibility, and adaptability. The project team was willing to work around the developing circumstances and needs of Instil Bio. This exceptional service played an integral role in the successful, on-time turnover of each phase.
Unique Challenges, Smart Solutions
Sometimes, ambitious projects may need carefully constructed solutions.
Challenge #1
The scope of work included the installation of all-new rooftop equipment, HEPA filters, cold room, tissue hub, lab-grade medical gas, and detection systems. Phases two and three were delivered during the peak of the COVID-19 pandemic and experienced global supply chain issues.
Solution
HITT collaborated with the client and the architect to identify alternate materials and/or equipment that better aligned with the project schedule and turnover milestones that could not be extended.
Challenge #2
Upon completion of the initial phase, the completed tumor hub immediately went into active use, with the transporting of live samples in and out of the facility.
Solution
The team closely coordinated the construction logistics, phasing, and access requirements to ensure there were no interruptions to the clinical trials occurring in the finished lab spaces.
Challenge #3
The rapid growth of the company created programmatic and design changes to make efficient use of the space and accommodate the new requirements. The phases were completed in an occupied space environment to allow the clinical trials and tests to continue uninterrupted.
Solution
The team remained very dynamic and fluid in our approach by understanding the modifications, and implementing the changes as quickly, efficiently, and safely as possible on-site. The project was completed on a double-shift schedule to meet clinical trial requirements.
The R&D Phase
The programming of the space is laid out in four quadrants to follow the lifecycle of clinical testing.



The Results
Tumor Hub and Office Space (ISO and clean room)
ISO-8 rated buildout including stainless steel doors, airlock, and HEPA fan filters.




Key Team Members
News & Insights
Let's chat.
Work With HITT
Learn More




